Predicting the Placebo response in OA to Improve the Precision of the Treatment Effect Estimation - Tools4Patient

A Prospective, Randomized, Open-Label Trial of Early versus Late Favipiravir Therapy in Hospitalized Patients with COVID-19 | Antimicrobial Agents and Chemotherapy

Supplemental Materials for Hepatic arterial infusion of oxaliplatin plus raltitrexed in patients with intermediate and advanced stage hepatocellular carcinoma: A phase II, single-arm, prospective study - European Journal of Cancer
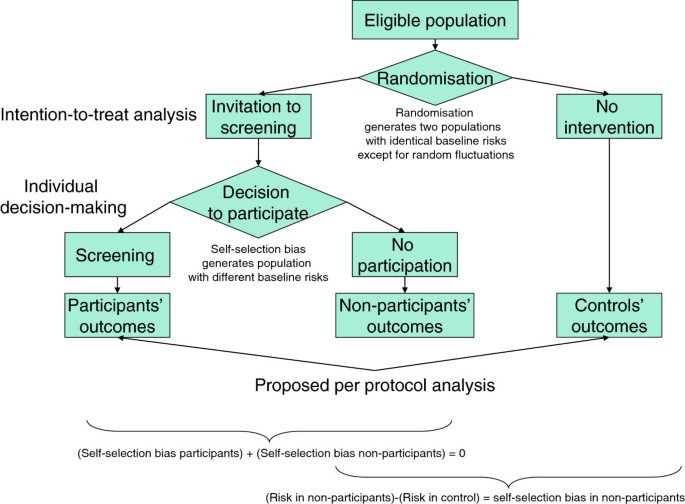
Screening: the information individuals need to support their decision: per protocol analysis is better than intention-to-treat analysis at quantifying potential benefits and harms of screening | BMC Medical Ethics | Full Text

Hydroxychloroquine in mild-to-moderate coronavirus disease 2019: a placebo-controlled double blind trial - Clinical Microbiology and Infection

Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18–59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial - The Lancet Infectious Diseases

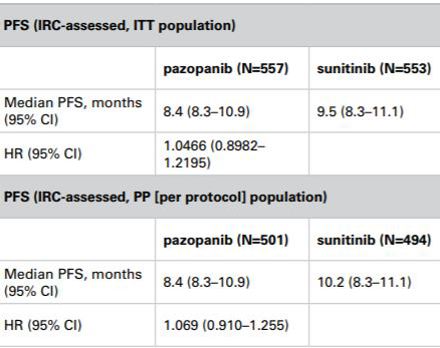
Discordance between reported intention-to-treat and per protocol analyses - Journal of Clinical Epidemiology

CONSORT Flow Diagram. *Per protocol population was used for applicable... | Download Scientific Diagram
A Randomized, Double-Blind, Efficacy and Safety Study of PF-05280586 (a Rituximab Biosimilar) Compared with Rituximab Reference