SOLVED:buffer solution is prepared as described in a) below: A solution of a strong base is then added to this buffer solution as described in part b): Note: the Ka of benzoic
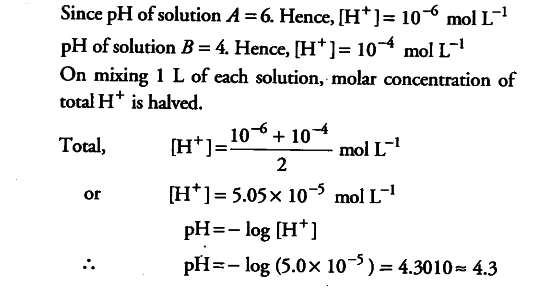
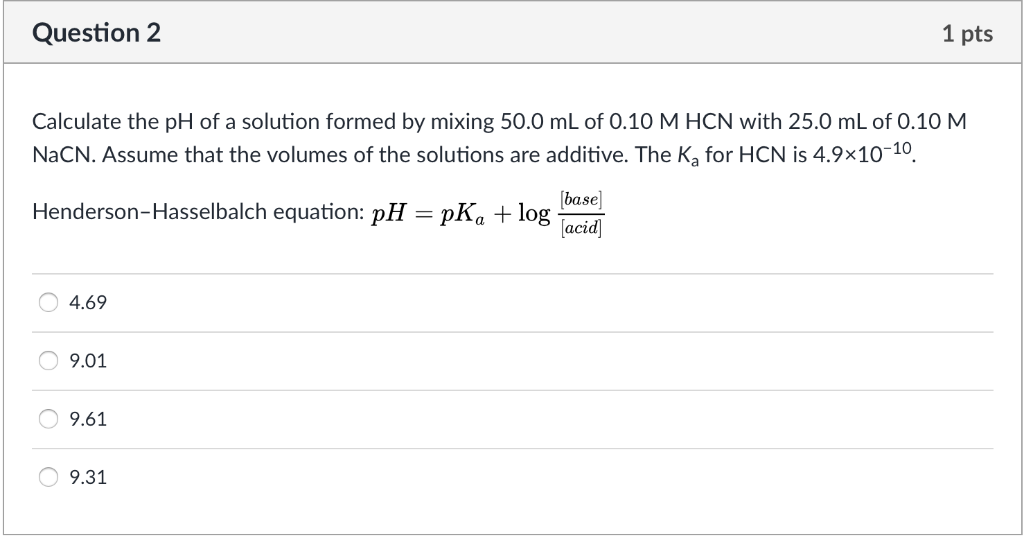
Calculate the pH of a solution formed by mixing equal volumes of two solutions, - CBSE Class 11 Chemistry - Learn CBSE Forum

ual volumes of following solutions are mixed, in which case the pH of resulting solution will be average value of pH of two solutions. (A) pH 2 HCI & pH 12 NaOH (

Q. 16. Calculate the pH of a solution formed by mixing equal volume of two solutions A and 8 of strong acids having pH-6 and pH-4 respectively.

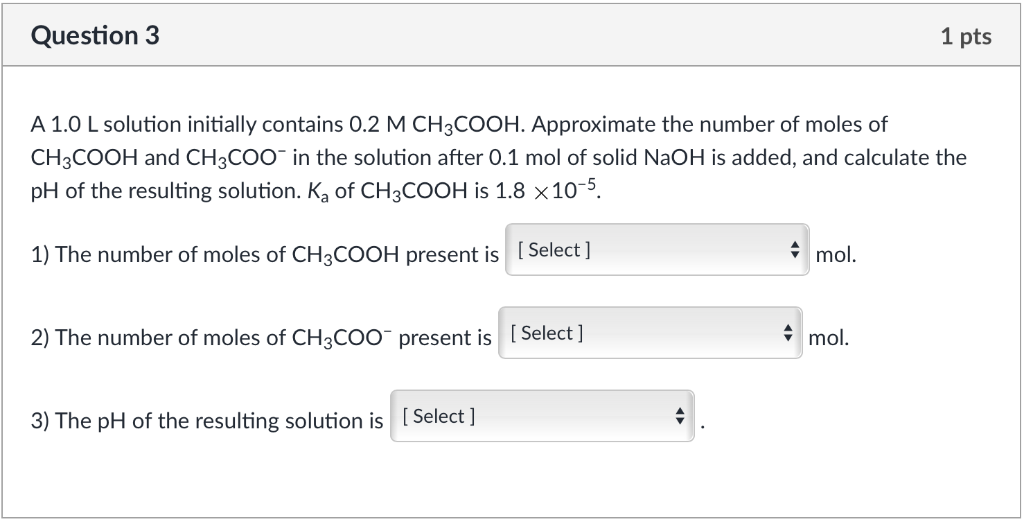
SOLVED:A buffer solution is prepared as described in a) below: A solution of a strong base is then added to this buffer solution as described in part b)_ Note: the Ka of
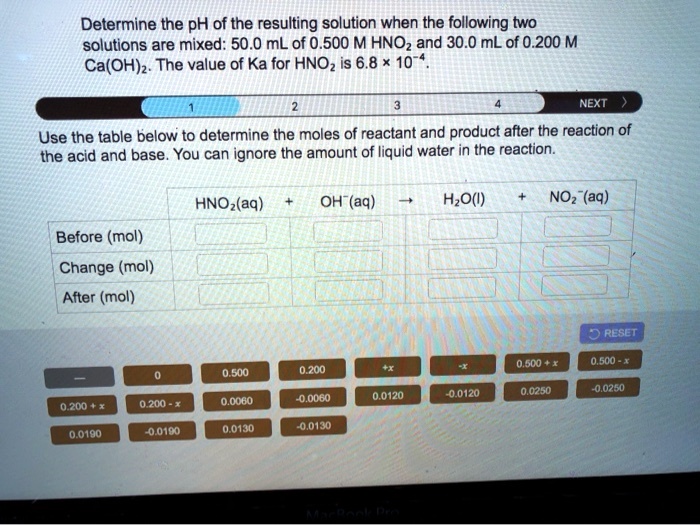
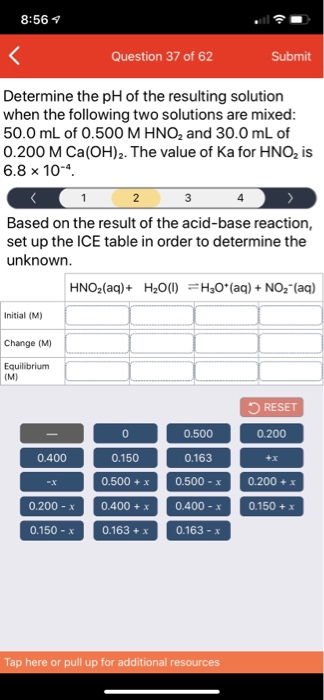
SOLVED:Determine the pH of the resulting solution when the following two solutions are mixed: 50.0 mL of 0.500 M HNOz and 30.0 mL of 0.200 M Ca(OH)z: The value of Ka for
The equal volume of two HCL solutions of pH=3 and pH=5 were mixed. What is the pH of the resulting solution? - Quora

54) The pH solution of obtained by mixing equal volumes of two solution of pH = 3 and pH = - Chemistry - Equilibrium - 10942093 | Meritnation.com

Equal volumes of two solutions having pH = 1 and pH = 4 are mixed together. The pH of the resulting solution is:

Equal volumes of two HCl solutions of pH = 3 and pH = 5 were mixed. What is the pH of the resulting solution?

1 Acid-base Theory 1.Arrhenius (1880s) : - Applied to aqueous solutions only Acids : Substances that produce hydrogen ions, H + (aq) when dissolved in. - ppt download